Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Retinal Alterations as Early Biomarkers of Neurodegenerative Diseases and Cognitive Impairment in The Older Adults
*Corresponding author: Mora Estrada Martha Regina, Medical Surgeon of the Faculty of Medicine, National Autonomous University of Mexico, University 1900, Romero de Terreros, Coyoacan, Mexico.
Received: October 17, 2022; Published:October 26, 2022
DOI: 10.34297/AJBSR.2022.17.002343
Abstract
Alterations in imaging studies of the retina, i.e., optical coherence tomography, fundus images, flowmetry and retinal oximetry are indicators of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, vascular dementia, amyotrophic lateral sclerosis, among others [1-4]. This imaging studies give an in vivo, non-invasive, and fast diagnostic alternative, which can potentially lead to treatment improvement and better preparation of the patients and their families to the severe stages of these diseases [5,6].
Keywords: Retina, Older adults, Cognitive Impairment, Neurodegenerative disease, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Schizophrenia, Multiple sclerosis, Vascular dementia, Lewy body dementia, Ganglion cells, Optical coherence tomography, Fundoscopy, Beta amyloid plaques, Age related macular degeneration
Introduction
The retina is the nervous layer of the eyeball, sharing its embryological origin with the central nervous system [1,7,8]. This opens a plethora of possibilities for evaluation of the brain and its alterations through retinal imaging, since it is a part of the central nervous system that can be seen in vivo, in a fast and noninvasive manner, using imaging studies such as optical coherence tomography, fundoscopy, doppler flowmetry and retinal pulse oximetry [2,7,9]. In these studies, we can evaluate alterations in vascularization, pathological protein accumulations, inflammation, changes in the thickness of retinal layers, among other data [2,5,10,11]. The neurodegenerative diseases in which these imaging methods have been applied to geriatric patients are Alzheimer’s disease, which is the leading cause of dementia, present in 10 to 15% of those over 65 years of age [9,12-15]; vascular dementia, which is the second cause of dementia [2,16-18]; Parkinson’s disease, which is the second most frequent neurodegenerative disease, present in approximately 1% of this population [3,10]. Other neurodegenerative diseases in which the usefulness of retinal studies has been demonstrated are amyotrophic lateral sclerosis [19-22], Lewy body dementia [3,12,23,24], multiple sclerosis [1,2,10,25,26], schizophrenia [1,27,28], and Huntington’s disease [4,29,30]. The study of retinal alterations could be an in vivo biomarker of these diseases, helping to establish the diagnosis as early as possible, which opens the door to timely care, delaying severe symptomatology and reducing complications, as well as creating new effective treatments [5,6]. It also provides the opportunity for the patient and family members to make decisions regarding housing, finding primary and auxiliary caregivers, financing, choice of treatment and advance directives before severe functional and cognitive impairments make it impossible for the patient to decide [5,6].
Methods
We used a systematic search in Pubmed, Google Scholar and Clinical Key literature databases under the concepts of: “Retina and cognitive deficiency”, “Retina and neurodegeneration”, “Macular degeneration and Alzheimer’s”, “Retina and Alzheimer’s”, “Retina and Parkinson’s”, “Retina and amyotrophic lateral sclerosis”, “Retina and dementia” taking into account only articles written between 2014 and 2019 in Spanish and English, including clinical studies, clinical trials, narrative reviews, meta-analyses and systematic reviews.
Content
The retina is the nervous layer of the eyeball and shares its embryological origin with the central nervous system [1,7,8]. Its formation starts from migratory pluripotential cells of the neuroectoderm of the diencephalic part of the neural tube, from which the central nervous system derives [1,7,31]. Because of this common origin, retinal microvasculature provides an insight into the cerebral vasculature [2,13,15]. Since retina and brain contain neurons, microglia, and astrocytes that confers them the same blood barrier [13,15,32]. Retina tissues are susceptible to changes when brain neurodegeneration occur [5,13,33] and can be an approach for the study, diagnosis, and prognosis in diseases such as Alzheimer’s, Parkinson’s, Lewy body dementia, amyotrophic lateral sclerosis, multiple sclerosis, schizophrenia, among others [1,4,10,20,27,34].
Alzheimer’s Disease
Since its first description in 1906 by the German psychiatrist Alois Alzheimer, ocular affections were observed in these patients, although usually attributed to damage in the visual cortex, numerous recent studies, and analyses [1,2,7,12,29,35] pointed out alterations in the retina that could be considered as biomarkers, since they manifest themselves before the memory deficit and cognitive deterioration [7,12,13]. The diagnosis of this disease is characterized by the formation of “senile plaques” of beta-amyloid protein and neurofibrillary tangles of tau protein in the brain found by necropsy [9,12,13,34]. Thus, retinal imaging methods are considered as an option for in vivo, noninvasive diagnosis of this disease [1,9,13,34,36].
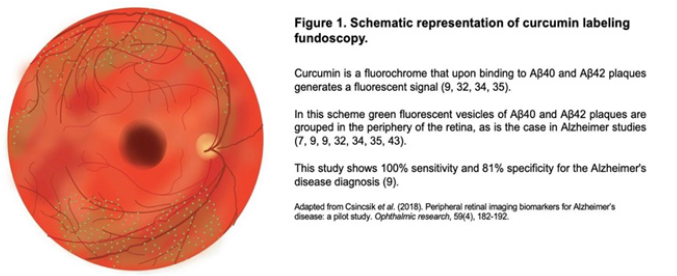
The first study to be discussed is fundoscopy with curcumin labeling, a fluorochrome that passes the blood-retinal barrier and upon binding to beta-amyloid plaques generates a fluorescent signal [9,29,32,34] (Figure 1). The presence of beta-amyloid protein and its amyloid precursor protein (APP) can be found as a normal part of aging [9,35], however neurotoxic proteins beta-amyloid protein40 (Aβ40) and beta-amyloid42 (Aβ42) aggregates are not found in geriatric patients without this disease [32,34,35,37-39]. These plaques have been found in the brain 15 to 20 years before the symptomatologic onset of Alzheimer’s disease, and studies have found these plaques in the retina years before in the brain, so they can be used as biomarkers leading to anti-amyloid treatments years before the onset of the brain disease [7,9,31,32,34,35,38]. Retinal Aβ40 and Aβ42 plaques, called “amyloid vesicles” [9], are associated to age-related macular degeneration, since in this disease we can find beta-amyloid protein within the drusen that characterize it [9,13,32,37,40,41]. The main difference is that in macular degeneration its drusen are found below the pigment epithelium in the macular region and there is disruption of Bruch’s membrane; while in Alzheimer’s disease the aggregates are found above the pigment epithelium in the periphery of the retina and Bruch’s membrane is intact [8,9,13,31,34,41-44] (Figure 1).
Accumulations of beta-amyloid protein in the retina cause a neuroinflammatory state increasing the expression of cytokines such as MCP-1, which stimulates apoptosis in the ganglion layer and gliosis [9,36,37]. Other cytokines such as IL-1β, IL-8, IL-3 damage the barrier properties of the pigment epithelium, leading to an inflammatory state, mitochondrial damage, accumulation of reactive oxygen species and hyperproduction of vascular endothelial growth factor [9,16,35]. Accumulation of Aβ40, Aβ42 and tau protein lead to neuronal and synapse loss in retinal layers, causing neurodegeneration [12,37,38,45]. Tau protein, which impairs synapses, mainly accumulates in the ganglion cell, inner plexiform, inner nuclear and outer plexiform layers, which can also be assessed by fundoscopy with fluorescent labeling [12,32,46].
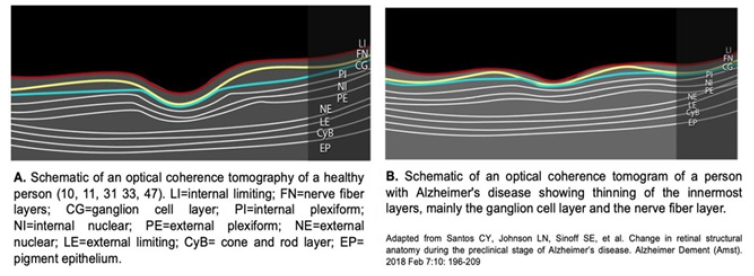
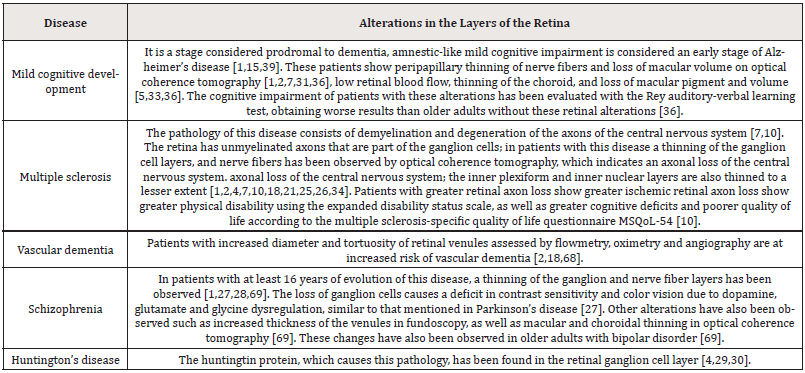
In Alzheimer’s disease, the first sign of disease in the retina is the thinning of the nerve fiber layer in the macular and peripapillary region [10,11,22,33,47], of at least 17. 5% (30), as the disease progresses, there is thinning in the internal limiting and ganglionic layers up to 25% at the level of the fovea [47], and in more advanced patients it has been observed that the thinning reaches the internal plexiform and internal nuclear layers [9,10,11,31,48]. These thinning is observed in optical coherence tomography, another in vivo, noninvasive, high-resolution method that allows measuring the thickness of the retinal layers and macular volume [2,5,31,46] (Figure 2).
The most frequently findings in different studies are the thinning of the ganglion cell and nerve fiber layers, optic nerve atrophy, reduction of macular volume and pigment, and choroidal thinning; these alterations become more evident as the disease progresses [2,9,11,13,29,33,36,43,47,49-55]. Patients with greater thinning of the ganglion cell and nerve fiber layers, with greater reduction in macular volume when subjected to the mini-mental examination scored 10 or lower, indicating severe cognitive impairment [11- 13,47]. Alterations in the retinal microvasculature in Alzheimer’s disease have been associated with increased memory impairment, decreased speed of thinking, and alterations in executive functions [2,56]. Alterations such as the reduction blood flow, smaller venular and arteriolar diameter due to accumulation of collagen and β-amyloid plaques in the vascular walls that increase the stiffness of their walls, and elevated oxygen saturation in venules and arterioles evaluated by oximetry [13,15,18,34,56].
In addition to the similarities noted between drusen in agerelated macular degeneration and amyloid vesicles in the retina in Alzheimer’s disease, there are other associations between the two diseases [16,37,40,57,58]. Patients with age-related macular degeneration have been found to be at increased risk for senile dementia, cognitive impairment and even Alzheimer’s disease, and this risk may be as high as 50% in individuals aged 69 to 97 years [16,37,40,59,60]. Six percent of patients with non-exudative age-related macular degeneration develop senile dementia or Alzheimer’s disease [16] and show greater cognitive impairment by the brief cognitive impairment test [59,60], poorer memory retention [59] and greater impairment in executive functions [16]. Betaamyloid plaques, contained in retinal drusen, stimulate an increase in vascular endothelial growth factor, increasing the risk of exudative macular degeneration in patients with Alzheimer’s disease [16]. Another interesting relationship between the two diseases is still under study; it has been observed that the administration of ranibizumab, an inhibitor of vascular endothelial growth factor, reduces the risk of senile dementia and Alzheimer’s disease [61].
Parkinson’s Disease
Retinal amacrine cells are interneurons that have synapses with the axons of bipolar cells and synapses with the dendrites of ganglion cells, these synapses are found in the inner plexiform layer of the retina [11,29,62]. Amacrine cell bodies and nuclei are in the inner nuclear layer of the retina and release dopamine, which confers contrast sensitivity and color vision, because retinal cells contain D1 and D2 receptors [11,29,63]. The pathophysiology of Parkinson’s disease consists of the loss of dopamine-producing cells and low concentrations of dopamine, mainly in the substantia nigra [3,11,29,64]. In the retina, loss of amacrine cells results in loss of dopamine and its functions, in addition to favoring accumulation of ubiquitin and α-synuclein, a protein associated with alterations in mitochondrial function and synaptic transmission [3,11,29,48,62]. Dopamine also regulates glutamate in the retina, so its deficit causes an elevation of glutamate, decreasing the synaptic reception capacity in the ganglion cells leading to nerve fibers atrophy that form the optic nerve [11,27]. The manifestations of this disease begin when 70-80% of the dopaminergic cells are lost, studies indicate that the loss of these cells before reaching these percentages, could give the opportunity to revert these effects with the administration of L-dopa, delaying and diminishing functional alterations in these patients [3,29].
The loss of retinal cellularity, particularly ganglion cells, can be assessed noninvasively by real-time imaging in a study called apoptotic retinal cell detection [3]. On optical coherence tomography, thinning of the macular region is evident mainly in the ganglion, inner plexiform, inner nuclear and outer plexiform layers [3,11,26,48,62]. It has been observed that the thinner the ganglionic and inner plexiform layers, especially at the macular level, the earlier and more severe the symptoms of the disease, this is associated with dopaminergic degeneration due to the loss of amacrine cells [3,23,29,62,64,65]. Fundoscopy with fluorescent labeling of αsynuclein, shows its accumulations in the inner nuclear and ganglion cell layers in Parkinson’s patients, especially in the retinal artery wall, these patients show greater impairment in hippocampal synaptic function and behavioral disorders [3,2,29,64].
Amyotrophic Lateral Sclerosis
Characterized by accelerated neurodegeneration of upper and lower motor neurons, causing muscle atrophy [19-21,56]. Patients usually die within three to five years after the onset of symptomatology, due to respiratory muscle paralysis, so it is of vital importance to detect this disease as early as possible [19,21,22,26]. Optical coherence tomography shows thinning in the nerve fiber, ganglion, inner plexiform, inner nuclear and outer plexiform layers, mainly in the macular area [20-22,26,34]. When cells of these layers are lost, several functions are lost: with the loss of bipolar cells of the inner nuclear layer, the transmission of color vision through synapses with ganglion cells is impaired, which has been proven by the Hardy-Rand-Ritter test [4,20,26], by losing amacrine and horizontal cells of the inner nuclear layer, which regulate synaptic transmission between bipolar and ganglion cells by inhibition with GABA, the amount of this neurotransmitter is reduced in the retina, in the same way that it has been reduced in the brain of patients with this disease [19].
As for markers, the autophagy marker p62 has been found in bipolar cells in perinuclear inclusions [26] in the plexus layers of the retina [26] in the inner and outer plexiform layers, which are the layers where synapses happen. Another autophagy marker has been found in high concentrations, ubiquitin 2. Ubiquitin 2 is believed to be causing ganglion cell death [26]. The loss of the nerve fiber layer, composed of ganglion cell axons, is caused by retrograde synaptic degeneration, like the degeneration of upper and lower motor neurons that occurs in this pathology; also, one eye is more affected than the contralateral eye, as occurs in the motor involvement of amyotrophic lateral sclerosis [4,19,26]. Patients with greater nerve fiber layer loss had a familial form of the disease and greater hippocampal synaptic dysfunction, which when evaluated by the revised functional assessment scale for amyotrophic lateral sclerosis and the Rankin scale, greater functional severity was found [4,20,26].
Lewy body dementia: The characteristic lesions of this disease are Lewy bodies and neurites, which are accumulations of αsynuclein [23,24]. These lesions can be evaluated by fundoscopy with fluorescent markings for α-synuclein, and are marked mainly in the outer plexiform, inner nuclear and ganglionic layers of the retina [23,24], these accumulations are associated with behavioral disorders [23,24]. Using an optical coherence tomography thinning in the nerve fiber layer is observed [12,18,66] in a comparative study with Parkinson’s disease and Alzheimer’s disease by Moreno Ramos T, et al. [67] where dementia due to Lewy bodies, presents the greatest thinning of this layer compared to the other two diseases. This thinning is associated with greater severity of dementia, since patients with these results obtained a severe deterioration in the Mattis dementia scale and in the mini-mental examination, compared with patients who did not show this thinning and their deterioration was mild [67] (Table 1).
Discussion
With the evidence demonstrated in the studies mentioned above, emphasizing those of Santos CY, et al. [7], Koronyo Y, et al. [32], Koronyo-Hamaoi M, et al. [34] and Csincsik L, et al. [43], it has been shown that it is possible to detect the changes of neurodegenerative diseases through the imaging study of the retina. Comparing normal aging changes with control groups, a significant difference is found. One limitation is that not all studies used scales such as mini-mental examination, Mattis dementia scale or some other method to assess the evolution of neurodegeneration and its symptomatology with respect to the changes observed in imaging studies. Studies such as Rohani M, et al. [4], Satue M, et al. [10], Den Haan J, et al. [11], Mukherjee N, et al. [21], Knoll B, et al. [36], Cunha LP, et al. [47], Al Salem KM, et al. (60), Moreno Ramos T, et al. [67] agreed that in imaging alterations the greater thinning of retinal layers according to the specific disease treated, the greater the evolution of neurodegeneration and therefore more severe symptomatology. It is worth mentioning that most of the studies referred to in this article do not mention the sensitivity and specificity of these diagnostic methods, so it is an important aspect that remains to be studied, because although it was shown that retinal manifestations can be found years before the symptomatologic onset of this group of diseases [3,5-7,9,12,13,29,31,32,34,35,38] the usefulness of these tests for early detection that can lead us to delay symptomatology, improve quality of life and even, in the future, implement new treatment options [5,6,61,68,69] still needs to be evaluated.
Conclusion
The study of the retina using imaging methods, such as fundoscopy and optical coherence tomography, is a noninvasive, in vivo, and rapid diagnostic aid for the neurodegenerative diseases mentioned in this article. Although its sensitivity and specificity has yet to be further evaluated, the retina opens possibilities for the early detection of these diseases. The benefits are the delay of severe symptomatology when treated early, preparation of the patient and family members for advanced stages, the opportunity for the patient to make decisions regarding treatment in severe stages, such as housing, care, financial and even the possibility of new treatment options in the future.
References
- Kwon JY, Yang JH, Han JS, Kim DG (2017) Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J Ophthalmol 31(6): 548-556.
- Sarah McGrory, James R Cameron, Enrico Pellegrini, Claire Warren, Fergus N Doubal, et al. (2016) The application of retinal fundus camera imaging in dementia: A systematic review. Alzheimers Dement (Amst) 6: 91-107.
- Eduardo Maria Normando, Benjamin Michael Davis, Lies De Groef, Shereen Nizari, Lisa A Turner, et al. (2016) The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson's disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathol Commun 4(1): 86.
- Mohammad Rohani, Alipasha Meysamie, Babak Zamani, Mohammad Mahdi Sowlat, Fahimeh Haji Akhoundi (2018) Reduced retinal nerve fiber layer (RNFL) thickness in ALS patients: a window to disease progression. J Neurol 265(7): 1557-1562.
- Eleonora M Lad, Dibyendu Mukherjee, Sandra S Stinnett, Scott W Cousins, Guy G Potter, et al. (2018) Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer's disease. PLoS One 13(2): e0192646
- Ferrari L, Huang SC, Magnani G, Ambrosi A, Comi G, et al. (2017) Optical Coherence Tomography Reveals Retinal Neuroaxonal Thinning in Frontotemporal Dementia as in Alzheimer's Disease. J Alzheimer’s Dis 56(3): 1101-1107.
- Cláudia Y Santos, Lenworth N Johnson, Stuart E Sinoff, Elena K Festa, William C Heindel, et al. (2018) Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement (Amst) 10: 196-209.
- Sha Sha Yu, Xin Tang, Yuen Shan Ho, Raymond Chuen Chung Chang, Kin Chiu (2016) Links between the brain and retina: the effects of cigarette smoking-induced age-related changes in Alzheimer’s disease and macular degeneration. Front Neurol 7:119.
- Sally S Ong, Alan D Proia, Heather E Whitson, Sina Farsiu, P Murali Doraiswamy, et al. (2018) Ocular amyloid imaging at the crossroad of Alzheimer’s disease and age-related macular degeneration: implications for diagnosis and therapy. J Neurol 266(7): 1566-1577.
- Maria Satue, Javier Obis, Maria J Rodrigo, Sofia Otin, Maria I Fuertes, et al. (2016) Optical coherence tomography as a biomarker for diagnosis, progression, and prognosis of neurodegenerative diseases. J Ophthalmol 2016: 8503859.
- Jurre den Haan, Lisanne J Balk, Frank D Verbraak (2018) Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol. 96(2): e265-e266.
- Kusne Y, Wolf AB, Townley K, Conway M, Peyman GA (2017) Visual system manifestations of Alzheimer's disease. Acta Ophthalmol 95(8): e668-e676.
- Garzón P SJ, Camacho M M, Tapiero L JA (2018) Características cognitivas y oculares en enfermedad de Alzheimer. NOVA. 16 (29): 101-114
- Alzheimer’s Association (2016) Alzheimer’s disease facts and figures. Alzheimers Dement 12(4): 459-509.
- Michael A Williams, Amy J McGowan, Chris R Cardwell, Carol Y Cheung, David Craig, et al. (2015) Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement 1(2): 229-235.
- Tsai DC, Chen SJ, Huang CC, Yuan MK, Leu HB (2015) Age-related macular degeneration and risk of degenerative dementia among the elderly in Taiwan: A population-based cohort study. Ophthalmology 122(11): 2327-2335.e2.
- Smith EE (2017) Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond) 131(11): 1059-1068.
- Lui Cheung CY, Ikram MK, Chen C, Wong TY (2017) Imaging retina to study dementia and stroke. Prog Retin Eye Res 57: 89-107.
- Hübers A, Müller HP, Dreyhaupt J, Böhm K, Lauda F, et al. (2016) Retinal involvement in amyotrophic lateral sclerosis: a study with optical coherence tomography and diffusion tensor imaging. J Neural Transm 123(3): 281-287.
- Marius Ringelstein, Philipp Albrecht, Martin Südmeyer, Jens Harmel, Ann Kristin Müller, et al. (2014) Subtle retinal pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 1:290-297.
- Nisha Mukherjee, Shan McBurney Lin, Anthony Kuo, Richard Bedlack, Henry Tseng (2017) Retinal thinning in amyotrophic lateral sclerosis patients without ophthalmic disease. PLoS One 12(9): e0185242.
- A Abdelhak, A Hübers, K Böhm, AC Ludolph, J Kassubek, et al. (2018) In vivo assessment of retinal vessel pathology in amyotrophic lateral sclerosis. J Neurol 265(4): 949-953.
- Diana L Price, Edward Rockenstein, Michael Mante, Anthony Adame, Cassia Overk, et al. (2016) Longitudinal live imaging of retinal α-synuclein: GFP deposits in a transgenic mouse model of Parkinson's Disease/Dementia with Lewy Bodies. Sci Rep 6: 29523.
- János Bencze, Viktória Simon, Erika Bereczki, Réka Majer, Gréta Varkoly, et al. (2017) [Clinical and neuropathological characteristics of dementia with Lewy bodies]. Orv Hetil 158(17): 643-652.
- Axel Petzold, Laura J Balcer, Peter A Calabresi, Fiona Costello, Teresa C Frohman, et al. (2017) Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 16(10): 797-812.
- Nicholas J Volpe, Joseph Simonett, Amani A Fawzi, Teepu Siddique (2015) Ophthalmic manifestations of amyotrophic lateral sclerosis (an american ophthalmological society thesis). Trans Am Ophthalmol Soc 113: T12.
- Pan J, Zhou Y, Xiang Y, Yu J (2018) Retinal nerve fiber layer thickness changes in Schizophrenia: A meta-analysis of case-control studies. Psychiatry Res 270: 786-791.
- Carlos Schönfeldt Lecuona, Thomas Kregel, Arno Schmidt, Elmar H Pinkhardt, Florian Lauda, et al. (2016) From imaging the brain to imaging the retina: optical coherence tomography (OCT) in Schizophrenia Schizophr Bull 42(1): 9-14.
- Chiara La Morgia, Fred N Ross Cisneros, Alfredo A Sadun, Valerio Carelli (2017) Retinal ganglion cells and circadian rhythms in alzheimer's disease, parkinson’s disease, and beyond. Front Neurol 8: 162.
- Borras García MR, Ondategui Parra JC, Ruiz Sáenz G (2016) Implicación visual de las enfermedades neurodegenerativas que cursan en alteración mental. Universidad politécnica de Cataluñ
- Jurre den Haan, Frank D Verbraak, Pieter Jelle Visser, Femke H Bouwman (2017) Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement 6: 162-170.
- Yosef Koronyo, David Biggs, Ernesto Barron, David S Boyer, Joel A Pearlman, et al. (2017) Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2(6): 93621.
- Gianluca Coppola, Antonio Di Renzo, Lucia Ziccardi, Francesco Martelli, Antonello Fadda, et al. (2017) Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One 10(8): e0134750.
- Maya Koronyo Hamaoui, Yosef Koronyo, Alexander V Ljubimov, Carol A Miller, Minhee K Ko, et al. (2011) Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54(1): S204-S217.
- JA Ratnayaka, LC Serpell, AJ Lotery (2015) Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond) 29(8): 1013-1026.
- Brianna Knoll, Joseph Simonett, Nicholas J Volpe, Sina Farsiu, Mallory Ward, et al. (2016) Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement 4: 85-93.
- Yuhai Zhao, Surjyadipta Bhattacharjee, Brandon M Jones, James M Hill, Christian Clement, et al. (2015) Beta-amyloid precursor protein (βAPP) processing in Alzheimer’s disease (AD) and age-related macular degeneration (AMD) Mol. Neurobiol 52(1): 533-544.
- Hernández Zimbrón LF, Martínez Hernández U, Pérez Hernández M, Abigail Torres Romero, Roberto Gonzalez Salinas, et al. (2018). Overproduction of different β-amyloid peptides in retina, optic nerve and visual cortex in healthy aging and alzheimer. Alzheimer's and Dementia 14(7): P353-P354.
- Mehmet Bulut, Aylin Yaman, Muhammet Kazim Erol, Fatma Kurtuluş, Devrim Toslak, et al. (2016) Choroidal Thickness in Patients with Mild Cognitive Impairment and Alzheimer’s Type Dementia. J Ophthalmol 2016: 7291257.
- Paul Mitchell, Gerald Liew, Bamini Gopinath, Tien Y Wong (2018) Age-related macular degeneration. The Lancet 392(10153): 1147-1159.
- Shaun Frost, Robyn Guymer, Khin Zaw Aung, S Lance Macaulay, Hamid R Sohrabi, et al. (2016) Alzheimer’s disease and the early signs of age-related macular degeneration. Curr Alzheimer Res 13(11): 1259-1266.
- Peep V Algvere, Anders Kvanta, Stefan Seregard (2016) Drusen maculopathy: a risk factor for visual deterioration. Act Ophthalmol 94(5): 427-433.
- Lajos Csincsik, Thomas J MacGillivray, Erin Flynn, Enrico Pellegrini, Giorgos Papanastasiou, et al. (2018) Peripheral retinal imaging biomarkers for Alzheimer’s disease: a pilot study. Ophthalmic Res 59(4): 182-192.
- Eichenbaum JW (2019) Key Transcription Factors Linking Macular Degeneration and Alzheimer’s Disease. W J Opthalmol & Vision Res 1(4): WJOVR.MS.ID.000516.
- Gao L, Liu Y, Li X, Bai Q, Liu P, et al. (2015) Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr 60(1): 162-167.
- Ana I Ramirez, Rosa de Hoz, Elena Salobrar Garcia, Juan J Salazar, Blanca Rojas, et al. (2017) The role of microglia in retinal neurodegeneration: alzheimer's disease, parkinson, and glaucoma. Front Aging Neurosci 9: 214.
- Leonardo Provetti Cunha, Luciana Cheker Lopes, Luciana Virgínia Ferreira Costa Cunha, Carolina Ferreira Costa, Leopoldo Antônio Pires, et al. (2016) Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in alzheimer's disease. PLoS One 11(4): e0153830.
- Elena Garcia Martin, Jose M Larrosa, Vicente Polo, Maria Satue, Marcia L Marques (2014) Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol 157(2): 470-478.
- Francisco J Ascaso, Nancy Cruz, Pedro J Modrego, Raul Lopez-Anton, Javier Santabárbara et al. (2014) Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol 261(8): 1522-1530.
- John M Nolan, Ekaterina Loskutova, Alan N Howard, Rachel Moran, Riona Mulcahy, et al. (2014) Macular pigment, visual function, and macular disease among subjects with Alzheimer’s disease: an exploratory study. J Alzheimer’s Dis 42(4): 1191-1202.
- Magda Gharbiya, Alessandro Trebbastoni, Francesco Parisi, Silvia Manganiello, Filippo Cruciani, et al. (2014) Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimer’s Dis 40(4): 907-917.
- Dachuan Liu, Lina Zhang, Zhen Li, Xuxiang Zhang, Yue Wu, et al. (2015) Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 15: 14.
- Alime Güneş, Seden Demirci, Levent Tök, Özlem Tök, Serpil Demirci (2015) Evaluation of retinal nerve fiber layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Turk J Med Sci 45(5): 1094-1097.
- V Polo, E Garcia Martin, M P Bamboz, J Pinilla, J M Larrosa, et al. (2014) Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer's disease. Eye 28(6): 680-690.
- Kelsey L Thomson, Jing Ming Yeo, Briony Waddell, James R Cameron, Suvankar Pal, et al. (2015) A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 1(2): 136-143.
- Martin R Turner, Michael Swash (2015) The expanding syndrome of amyotrophic lateral sclerosis: a clinical and molecular odyssey. J Neurol Neurosurg Psychiatry 86(6): 667-673.
- Shiu Dong Chung, Cha Ze Lee, Li Ting Kao, Herng Ching Lin, Ming Chieh Tsai, et al. (2015) Association between neovascular age-related macular degeneration and dementia: a population-based case-control study in Taiwan. PLoS One 10(3): e0120003.
- Cecilia S Lee, Eric B Larson, Laura E Gibbons, Aaron Y Lee, Susan M McCurry, et al. (2019) Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement 15(1): 34-41.
- Luca Rozzini, Maddalena Riva, Nausica Ghilardi, Paola Facchinetti, Eliana Forbice, et al. (2014) Cognitive dysfunction and age-related macular degeneration. Am J Alzheimers Dis Other Demen 29(3): 256-262.
- Khalil M Al-Salem, Shlomit Schaal (2014) Mini-cognitive testing in patients with age-related macular degeneration. Retina 34(5): 868-873.
- Tiarnan DL Keenan, Raph Goldacre, Michael J Goldacre (2014) Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol 132(1): 63-68.
- Esin S Sari, Rabia Koc, Alper Yazici, Gözde Sahin, Sitki S Ermis (2015) Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J Neuroophthalmol 35(2): 117-121.
- Ji Guo Yu, Yi Fan Feng, Yi Xiang, Jin Hai Huang, Giacomo Savini, et al. (2014) Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PloS One 9(1): e85718.
- Jessica Chorostecki, Navid Seraji Bozorgzad, Aashka Shah, Fen Bao, Ginny Bao, et al. (2015) Characterization of retinal architecture in Parkinson’s disease. J Neurol Sci 355(1-2): 44-48.
- Beatriz Jiménez 1, Francisco J Ascaso, José A Cristóbal, Javier López del Val (2014) Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord 29(1): 68-74.
- Elena Garcia Martin, Vicente Polo, Jose M Larrosa, Marcia L Marques, Raquel Herrero, et al. (2014) Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology 121(2): 573-579.
- Teresa Moreno Ramos, Julián Benito León, Alberto Villarejo, Félix Bermejo Pareja (2013) Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis 34(3): 659-664.
- Victor T T Chan, Tiffany H K Tso, Fangyao Tang, Clement Tham, Vincent Mok, et al. (2017) Using Retinal Imaging to Study Dementia. J. Vis. Exp (129): 56137.
- Peter Joe, Meleha Ahmad, Gabriella Riley, Judith Weissman, R Theodore Smith, et al. (2018) A pilot study assessing retinal pathology in psychosis using optical coherence tomography: Choroidal and macular thickness. Psychiatry Res 263: 158-161. a href="https://pubmed.ncbi.nlm.nih.gov/1184598/">N Akkas (1975) Dynamic analysis of a fluid-filled spherical sandwich shell-A model of the human head. J Biomech 8(5): 275-284.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.